Patient Focus
Today’s clinical development is more patient-centric – focusing not only on treating the patient’s disease or dysfunction but also on the patient’s experience of, and desired outcomes for, treatment. Sponsors must make mindful decisions as they select new technologies to directly collect data from patients and use it in statistical analyses. Multiple parties are involved in the decision around collecting patient-reported outcomes (PROs), one of the four forms of clinical outcomes assessment (COA). Drug and device development sponsors, sites, CROs, patients, and even patients’ families have roles in determining what PRO tools are best suited to a particular study. PROMETRIKA’s data management experts have eased sponsors’ entries into the effective use of ePRO tools in today’s electronic data environment.
Evolution of an Instrument
Beginning in the early 2000’s, to try to mitigate some of the drawbacks of collecting data on paper PRO instruments, some sponsors switched to PROs reported on dedicated electronic hand-held devices. While this approach solved some of the problems of paper reporting, new challenges developed. The need to load the program on each piece of hardware individually, provide hardware repair/replacement, collect the hardware, and download the data at the end of the trial, made this an expensive option. Subsequently, the use of cell phones and the spread of wifi made wireless communication commonplace and opened the door for an easier electronic means of collecting PROs. Today, many sponsors use these available technologies in clinical studies.
A Challenge
Recently, one of PROMETRIKA’s sponsors approached us with a challenge. The sponsor was enthusiastic about using cutting-edge ePRO tools to directly capture patient experiences but was concerned that the solutions would be far more costly than the paper formats they had used in the past. Additionally, the sponsor was worried about the time commitment necessary to coordinate the services of ePRO, EDC, and device providers. We led the sponsor through several cost scenarios and came up with a solution that fit the sponsor’s budget. In this case, to alleviate the cost of device purchases, we proposed that only site-based devices be used and that patients would complete the ePRO immediately after study drug dosing at the study visit. This was also effective for ensuring that timely, complete data were obtained; study personnel were available to guide the patient and make certain that all necessary data were entered before the patient left the site. PROMETRIKA managed configuration of devices, distribution to the site, site training, and ultimate integration of ePRO data with the electronic database.
Applied Expertise
Some sponsors are still concerned that the use of electronic devices may be particularly difficult for certain patients such as the elderly or physically challenged. However, some research has shown that electronic diary completion has a 95% compliance rate among the elderly. Technology can be scary to some people, but we can help sponsors find study-appropriate solutions that improve the likelihood of successful ePROs implementation.
Continuing the Conversation
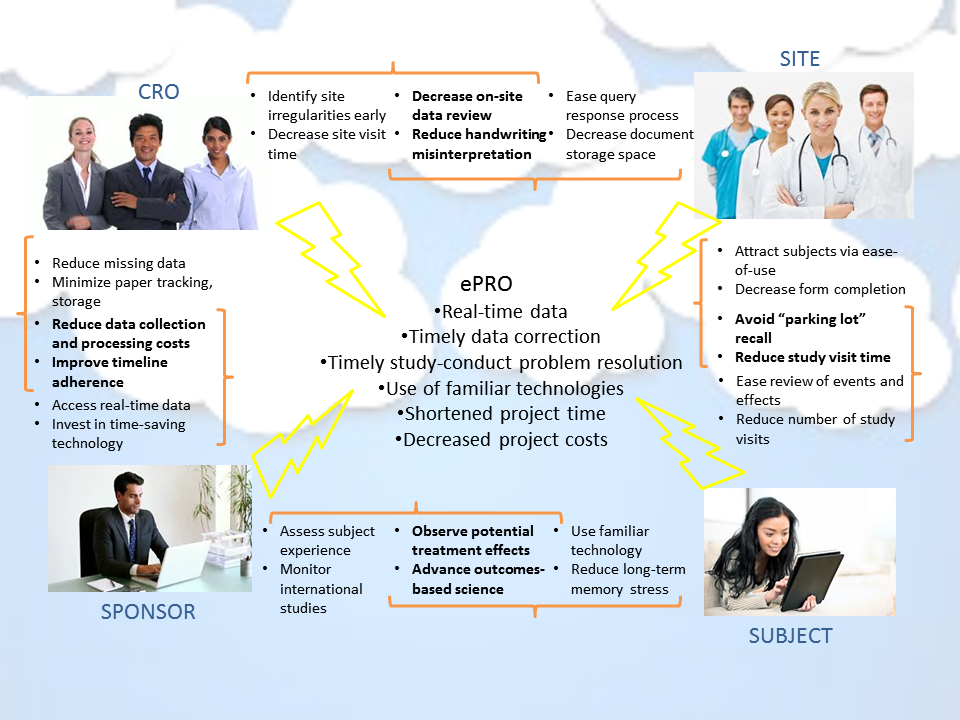
The use of ePRO tools and their integration with EDC systems have advantages to all the parties involved in a clinical trial. As shown in the diagram below, sponsors, sites, CROs, and subjects have independent AND overlapping needs that can be met with ePRO solutions. In upcoming blogs, PROMETRIKA will discuss the many steps and considerations in selecting and implementing an ePRO solution, such as:
- In which therapeutic areas does ePRO collection make the most sense?
- In which patient populations should an at-home vs. in-office solution be implemented? What size of device is best?
- How much time does a sponsor need to make a decision to use ePRO tools, to select those tools, and to have those tools integrated with an EDC system?
- What roles and functions need to be involved in selecting PRO tools and configuring them for electronic use?
- What input and support should a sponsor expect from a CRO, ePRO vendor, EDC vendor, and device provisioning company when planning an ePRO solution?
- How can study sites be prepared to use ePROs? What training is required and who can provide that training? What continued support is necessary?
- How can a sponsor leverage the expertise and cross-disciplinary resources of a CRO?
PROMETRIKA asks: What challenges are sponsors and providers facing when selecting and implementing ePRO solutions?