All team members at PROMETRIKA take responsibility for remaining well versed in the changes in regulations and trends that impact their work. As a biostatistician at PROMETRIKA, I recently attended the American Statistical Association annual Biophamaceutical Section Regulatory-Industry Statistics conference where we discussed the addendum to the E9 (R1) Statistical Principles for Clinical Trials titled “Estimands and Sensitivity Analysis in Clinical Trials.” The addendum introduces the Estimands Framework and strategies for selecting an estimand. PROMETRIKA invites you to explore this addendum and learn about the estimand strategies put forth in this update. PROMETRIKA can assist you with these and other clinical trial needs.
A Brief Overview of the Estimands Framework Addendum: Choosing the Best Strategy
In an ideal world, all participants in a randomized clinical trial would complete a trial exactly per the protocol, with no incomplete data. As anyone involved with clinical trials knows, this is rarely the case. In reality, there are many events that can impact the collection and measurement of the outcome of interest and, therefore, challenge the interpretation of the treatment effect.
To address some of these concerns, in May 2021, the FDA released an addendum to the E9(R1) Statistical Principles for Clinical Trials entitled “Estimands and Sensitivity Analysis in Clinical Trials” with the purpose to provide a uniform framework to “align planning, design, conduct, analysis and interpretation for clinical trials.”
This addendum aims to target issues with the current practice of defining the clinical trial objectives and the treatment effects. The goal is to ensure the target of estimation is clearly defined and easily interpretable in the protocol and statistical analysis plan (SAP). This framework aims to increase the reproducibility of clinical trial results and transparency in communicating the efficacy of treatments. Although including estimands in protocols and SAPs are not currently required, adopting the Estimands Framework in trial planning will ensure statistical analyses are consistent with the treatment effect of interest and potentially comply with future regulations.
The framework describes the importance of defining estimands and strategies to address intercurrent events (ICE) as described below.
What is an estimand?
An estimand is a precise description of the treatment effect being estimated. The ICH E9 (R1) addendum reiterates that an estimand “summarizes at the population level what the outcomes would be in the same patients under different treatment conditions being compared.” This should be reflected in the clinical question the analysis is answering and selected based on the disease setting and aim of treatment. Estimands address how ICEs are handled in the study, and should be constructed prior to choices about data collection and analysis methods.
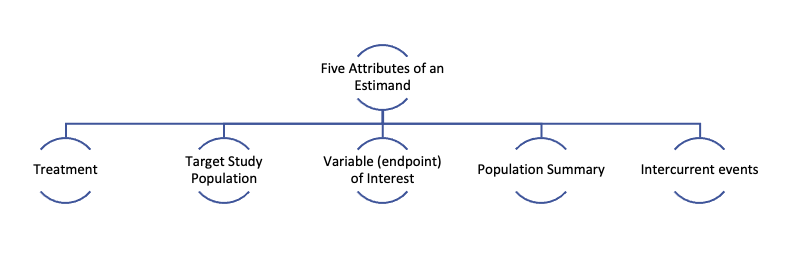
An estimand has five attributes as indicated in the figure below. These five inter-related attributes help clarify the clinical question of interest.
What are Intercurrent Events?
The addendum also introduced the concept of ICEs which are events occurring after treatment initiation that affect either the interpretation or the existence of the measurements associated with the clinical question of interest. Examples include treatment discontinuation for an adverse event, patient death on study or use of a rescue medication. It is important to note that study discontinuation, loss-to follow-up and other missing data are not considered ICEs.
Estimand Strategies
There are five strategies identified in the E9(R1) to address ICEs. These include: Treatment-Policy, Composite, Hypothetical, Principal Stratum, and While-on-Treatment (additional details below). When choosing a strategy, it is imperative to have input from clinicians, statisticians, sponsors and regulators. The strategy should be chosen based on each ICE to reflect the clinical question of interest along with the therapeutic and experimental context. These strategies can be used separately or combined to address multiple types of ICEs.
|
Definitions of Estimand Strategies from ICH E9 (R1) |
||
|---|---|---|
Strategy |
Description |
Example |
|
Treatment-Policy Strategy |
The data collected for the variable of interest are used regardless of whether or not the ICE occurs. |
When a patient uses a rescue medication, the data collected after the event is used regardless of rescue medication use. |
|
Composite |
The occurrence of the ICE is taken to be a component of the variable. |
If a patient takes a rescue medication, the event (use or non-use) is incorporated into the endpoint. |
|
Hypothetical |
A hypothetical scenario is envisioned in which the ICE would not occur. |
The endpoint considered would be predicted or imputed as though patients had not had a rescue medication available. This must be used with care only in relevant situations as it is based on assumptions. |
|
Principal Stratum |
Assessing the treatment effect in the strata of patients defined by the potential of ICEs on either or both treatments. |
The stratum of patients who are not likely to need a rescue medication would be assessed. The stratum of patients would be defined prior to the start of the trial. |
|
While on Treatment |
Response to treatment prior to the occurrence of the ICE is of interest. |
Only data collected prior to the use of a rescue medication would be assessed. |
References
Clark TP, Kahan BC, Phillips A, et al. Estimands: bringing clarity and focus to research questions in clinical trials. BMJ Open. 2022;12:e052953. doi: 10.1136/bmjopen-2021-052953.
Gogtay NJ, Ranganathan P, Aggarwal R. Understanding estimands. Perspect Clin Res. 2021 Apr-Jun;12(2):106-112. doi: 10.4103/picr.picr_384_20. Epub 2021 Mar 12. PMID: 34012908; PMCID: PMC8112325.
ICH E9(R1) Expert Working Group. ICH E9(R1) Estimands and Sensitivity Analysis in Clinical Trials. 2021 Dec.
Ionan AC, Mayo S, Paterniti M, Scott J. “Estimand Framework Implementation”. ASA Biopharmaceutical Section Regulatory-Industry Statistics Workshop. 2022 Sep 20.
Kahan, B.C., Morris, T.P., White, I.R. et al. Estimands in published protocols of randomised trials: urgent improvement needed. Trials. 2021;22:686. Accessed Nov 2, 2022. https://doi.org/10.1186/s13063-021-05644-4.
Mitroiu, M., Oude Rengerink, K., Teerenstra, S. et al. A narrative review of estimands in drug development and regulatory evaluation: old wine in new barrels?. Trials. 2020;21:671. Accessed Nov 2, 2022. https://doi.org/10.1186/s13063-020-04546-1.


