The accurate analysis and reporting of data is necessary to the evaluation of new treatments for human diseases. Regulatory authorities must weigh the risks with the benefits of treatments in their approval decisions. Often, regulators will ask sponsors to provide information about the analyses, such as the datasets and data selection criteria used to generate the results. The Clinical Data Interchange Standards Consortium (CDISC) has described their newest initiative to standardize analysis results in the form of tables, listings, and figures (TLF) and reporting of data across the industry.
The Analysis Results Standard (ARS) will provide a standard format for linking results with the data used to generate them, allowing regulatory reviewers access to important information about the analyses; a ‘breadcrumb trail’ from data collection to data aggregation to analysis and results. An implementation guide will outline how to standardize analysis result datasets to enable automation of TLF production. This will facilitate easy storage, access, processing, and reproduction of analysis results, improving reusability of analyses, and ensuring traceability to the Protocol, SAP and input data.
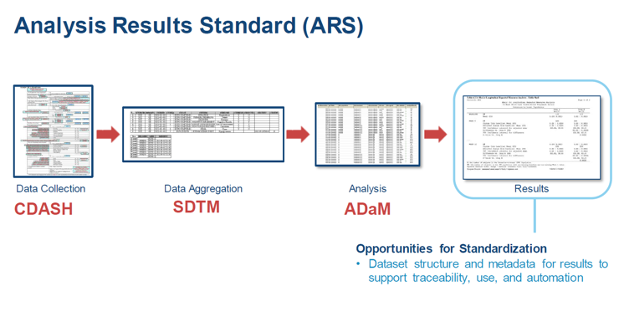
At present, sponsor companies spend a considerable amount of time manually designing TLF shells and writing analysis dataset specifications. Programmers then write SAS code to generate analysis datasets and TLFs. This process is time-consuming even with the use of macros or by reusing previously generated code.
The planned outcomes of the ARS initiative are the Analysis Results Metadata Technical Specification (ARM-TS) and the Analysis Results Dataset (ARD). ARM-TS would define metadata needed to generate statistical analysis results and associated displays, while ARD would be used as a standardized format for producing statistical analysis results in a consistent manner. In conjunction with these guidelines, CDISC plans to create an open-source tool that can design TLFs and generate the associated metadata.
CDISC has already released the ARS and its user guide for public review and is currently resolving the resulting comments. Members of PROMETRIKA’s team of statistical programmers had the opportunity to attend a hands-on session with early-model prototypes. They gained valuable insights into the ARS objectives and felt like they were part of the development of a truly innovative tool.
PROMETRIKA’s statistical programmers and biostatisticians are always learning and implementing the latest industry practices to enhance our sponsors’ reports and submissions. When the final, validated ARS tools become available, the team will be investigating these methods to potentially integrate into our processes, with the aim of saving our sponsors’ time and cost in meeting regulators’ data evaluation needs.


